The Bluebelle Study: a feasibility study of three wound dressing strategies in elective and unplanned surgery
Study summary
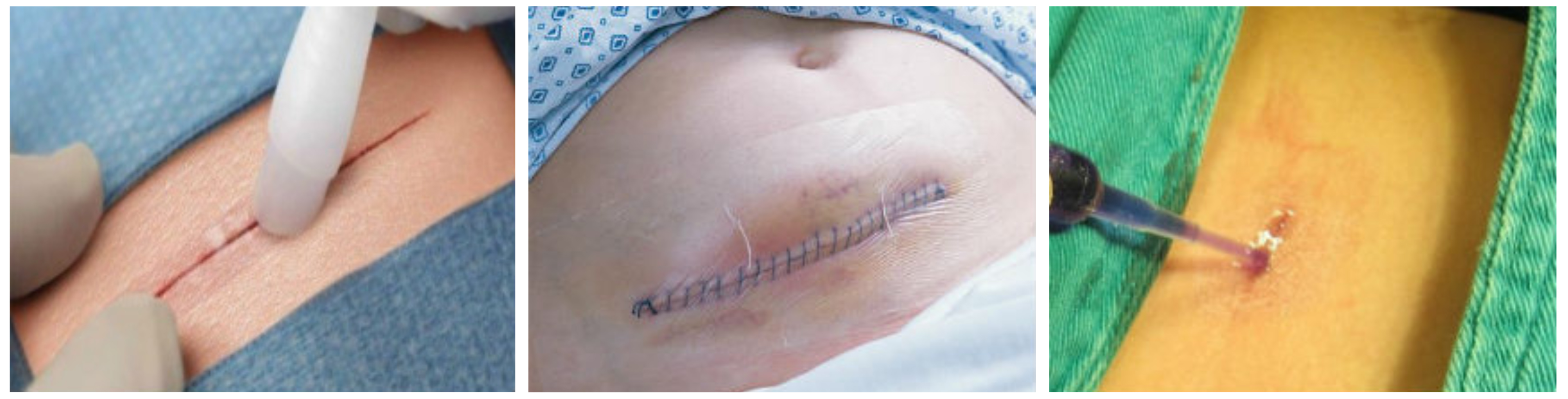
Up to a quarter of operations performed in England are complicated with surgical site infections (SSIs), and whilst many resolve with simple treatment, some cause morbidity and major costs for the NHS. Minimising the risk of developing an SSI is therefore imperative. One area of controversy is the role of wound dressings, which are applied as standard practice following surgery in adults, but rarely in children. A systematic review of previous studies showed no evidence to suggest that using a dressing reduces the risk of SSIs, or that any particular dressing is more effective at reducing scarring, controlling pain or promoting patient acceptability. However, most studies were small and at high risk of bias, thus the quality of the evidence is poor.
The Bluebelle Study was an NIHR Health Technology Assessment (HTA)-funded feasibility study carried out in two parts.
Phase A (July 2014 to March 2016) included: interviews with surgeons, nurses, midwives and adult patients to investigate current use of dressings and views about not using dressings; development of assessment measures for wounds and SSIs for use by clinicians and patients; observation of wound closure in theatre and literature reviews.
Phase B (February 2016 to October 2016), involved a pilot randomised controlled trial (RCT) to establish whether a larger RCT comparing the cost-effectiveness of simple dressing, tissue adhesive used as a dressing and no dressing to reduce SSIs following elective and unplanned surgery was possible. Phase B examined the overall acceptability of trial participation and delivery, and provided the opportunity to validate the new SSI outcome measure developed in Phase A.
Chief Investigator
Jane Blazeby, University of Bristol
Study Coordinator and Primary Contact
Bristol Trials Centre (CTEU)
Email: bluebelle-study@bristol.ac.uk




