A more complete understanding
Dr Bethan Lloyd-Lewis, Vice Chancellor’s Fellow at the School of Cellular and Molecular Medicine at the University of Bristol, is investigating the link between breast development and breast cancer using several different approaches, as she explains:
“My research aims to develop a more complete understanding of the cellular and molecular mechanisms underpinning the relationship between breast development and the risk of breast cancer,” Dr Lloyd-Lewis said.
“Breast development is driven by stem/progenitor cells,” she continued, “which are considered possible cells-of-origin in some breast cancers. In certain contexts, committed (more ‘differentiated’) cells are plastic – they can ‘de-differentiate’, or change to a more primitive cell-type. This might represent an early step in breast cancer. It’s also possible that the behaviours of these cells during breast development can predispose women to breast tumours later in life”.
Age and reprogramming
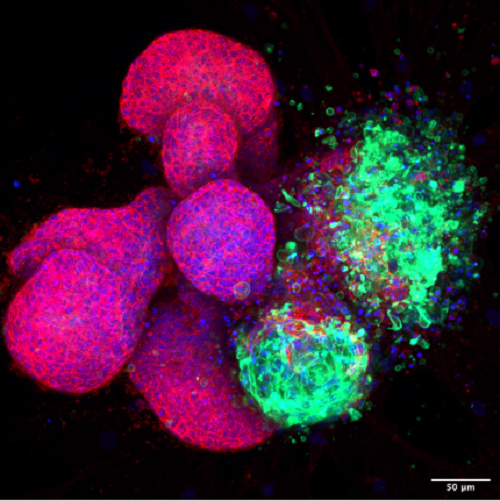
The first thing Dr Lloyd-Lewis and her team needed to do was to understand what age and developmental stages such as puberty and pregnancy do to affect the mammary (breast) cells’ ability to reprogramme themselves. Using a grant from the Academy of Medical Sciences, with help from the Elizabeth Blackwell Institute COVID-19 Support Scheme for Early Career Researchers, Dr Lloyd-Lewis’ work involves investigating the molecular mechanisms driving reprogramming using genetically altered pre-clinical mouse and mammary organoid culture models. These models enable the tagging and tracking of specific mammary cell types within the tissue, and by looking at different ages and developmental stages, the team can compare the plasticity of distinct mammary cell populations.
New approaches and findings
“Firstly, we had to develop methods of inducing plasticity of mammary cells in our models. Previous work had shown that DNA damage can induce cellular plasticity in different tissues. We first used radiation as an approach, which didn’t work as well as we’d hoped, so we now use cisplatin (a drug that causes DNA damage) which was more efficient at inducing the changes we were looking for.”
First of all, Dr Lloyd-Lewis and her team compared the plasticity of different mammary cells at puberty and early adulthood. They are now investigating the impact of age and parity on cellular plasticity. “Our plasticity experiments are still ongoing,” said Dr Lloyd-Lewis, “ We have interesting new observations on the plasticity of some of these cells in puberty compared to adulthood; which my team and I are further investigating using a variety of experimental approaches in mammary organoids, using a 3D culture system that I developed earlier in my career.”
Funding meant additional time
“My Academy of Medical Sciences Award project was substantially delayed thanks to COVID-related closures at the University”, said Dr Lloyd-Lewis. “The funding from the Elizabeth Blackwell Institute has been instrumental in helping me make up for lost time, and has extended the time I have technical assistance in the lab, which has been enormously beneficial. I have been able to maintain my research activity while having more time to write grant applications and manage my interdisciplinary collaborations with researchers at Bristol University’s Medical Research Council Integrative Epidemiology Unit, which are already bearing fruit.
Dr. Bethan Lloyd-Lewis, Vice-Chancellor’s Research Fellow at the School of Cellular and Molecular Medicine (CMM), has been awarded a £3.1 million fellowship from the Wellcome Trust to lead research into the regulatory mechanisms influencing stem cell fate decisions during organ development and cancer.
"I’m incredibly grateful to the Elizabeth Blackwell Institute for supporting my research. The funding had been pivotal for advancing the development of our laboratory models and refining approaches applicable to a spectrum of projects in my lab. This has significantly strengthened my grant applications, including my recent successful application for a Wellcome Trust Career Development Award.”
Dr Lloyd-Lewis secured the esteemed 8-year Wellcome Trust Career Development Award, which recognises mid-career researchers who have the potential to be international research leaders. This award aims to support researchers in driving ambitious programs of work that lead to substantial shifts in understanding that could have significant implications for human health. Read more about this news