Receptors
Glutamate receptors come in many flavours!
Glutamate receptors come in many flavours - that is there are many different types and sub-types.
Ionotropic receptors
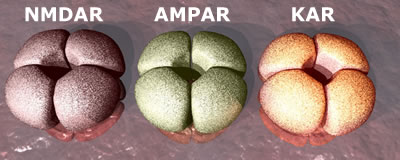 There are three types
of ion channel receptor, named after chemicals that specifically interact
with them - NMDA, AMPA and Kainate receptors. All the ion channel receptors have a similar structure. They are built from assemblies of four separate proteins (subunits) that make a channel or pore through the membrane. It is through this pore that ions can pass. The subunits themselves come in different variants. For instance, the NMDA receptor (NMDAR) is built from GluN1 subunits (required) as well as subunits chosen from GluN2A, B,C or D. Thus the receptor composition can be GluN1:GluN2A, GluN1:GluN2B, GluN1:GluN2AB ... etc. In addition, the subunits come in
multiple forms - so the number of possible combinations of individual subunits is astronomical! Each combination of receptor subunits can bring subtle variations in the properties of those receptors and so although we may talk about 'the NMDA receptor', what we really mean is a family of receptors that may react differently to the same signal in different neurons and/or at different stages in development.
There are three types
of ion channel receptor, named after chemicals that specifically interact
with them - NMDA, AMPA and Kainate receptors. All the ion channel receptors have a similar structure. They are built from assemblies of four separate proteins (subunits) that make a channel or pore through the membrane. It is through this pore that ions can pass. The subunits themselves come in different variants. For instance, the NMDA receptor (NMDAR) is built from GluN1 subunits (required) as well as subunits chosen from GluN2A, B,C or D. Thus the receptor composition can be GluN1:GluN2A, GluN1:GluN2B, GluN1:GluN2AB ... etc. In addition, the subunits come in
multiple forms - so the number of possible combinations of individual subunits is astronomical! Each combination of receptor subunits can bring subtle variations in the properties of those receptors and so although we may talk about 'the NMDA receptor', what we really mean is a family of receptors that may react differently to the same signal in different neurons and/or at different stages in development.
Metabotropic Receptors
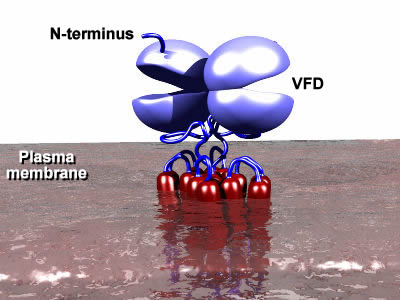 In addition,
there is another group of glutamate receptors that are not ion channels,
but pass signals into the cell via other accessory proteins (G-proteins).
These receptors are termed the metabotropic
glutamate receptors. There are several families of such receptors (also termed G-protein coupled receptors or GPCRs) and the metabotropic glutamate receptors (mGluRs) belong to a family that also includes taste receptors and several hormone receptors that are distinct from the prototypical family that contains receptors such as the dopamine receptors, muscarinic receptors and rhodopsin. One of the main distinguishing features is the Venus Flytrap Domain (VFD) that forms the binding site for the neurotransmitter (or hormone ... or whatever you are tasting!). This domain is found at the external face of the plasma membrane. As the name suggests, it acts like a venus flytrap, closing once the right molecule has entered and latched onto its binding site. This in turn changes the shape of the rest of the protein, triggering the release of the G-proteins that are bound to the receptor on the internal face of the membrane. The release of the G-protein initiates a signaling cascade that results in functional changes inside the cell. Thus the binding of glutamate to the outside of the neuron affects what happens inside that neuron, but in an indirect manner.
In addition,
there is another group of glutamate receptors that are not ion channels,
but pass signals into the cell via other accessory proteins (G-proteins).
These receptors are termed the metabotropic
glutamate receptors. There are several families of such receptors (also termed G-protein coupled receptors or GPCRs) and the metabotropic glutamate receptors (mGluRs) belong to a family that also includes taste receptors and several hormone receptors that are distinct from the prototypical family that contains receptors such as the dopamine receptors, muscarinic receptors and rhodopsin. One of the main distinguishing features is the Venus Flytrap Domain (VFD) that forms the binding site for the neurotransmitter (or hormone ... or whatever you are tasting!). This domain is found at the external face of the plasma membrane. As the name suggests, it acts like a venus flytrap, closing once the right molecule has entered and latched onto its binding site. This in turn changes the shape of the rest of the protein, triggering the release of the G-proteins that are bound to the receptor on the internal face of the membrane. The release of the G-protein initiates a signaling cascade that results in functional changes inside the cell. Thus the binding of glutamate to the outside of the neuron affects what happens inside that neuron, but in an indirect manner.
The signaling cascades activated by these receptors can be very long and complex and so the resulting changes in function are relatively slow. Thus, mGluRs are not involved directly in neurotransmission - that is the domain of the ionotropic receptors (iGluRs). However, the mGluRs regulate the operation of the iGluRs - they are involved in determining how many iGluR receptors are present in the post-synaptic part of the synapse and in how well they allow ions through their channels. In this way, they can determine how strong the response is to a given signal, acting like a volume or gain control on an amplifier. They are also found in the pre-synaptic elements of the synapse where they can determine how much neurotransmitter is released into the synaptic cleft and so affecting the size of the initial signal as well. Interestingly, this means that the mGluRs can regulate the release of neurotransmitters other that glutamate ... indeed they have been shown to regulate the release of dopamine (important in the reward systems in the brain and in the control of complex movement) and of GABA, the major inhibitory neurotransmitter.
Further detailed information on the structure and function of these receptors is available.

