The term ion channels is often used
in text books and by students to refer to those channels gated
by voltage. It is better that you use the term voltage-gated
ion channel if that is what you mean. They form a superfamily
of voltage-gated ion channels that share a common structure and
which have evolved one from the other. This is the main reason
why it is so difficult to develop new drugs that are specific
for only one type of ion channel - their relatedness makes it
likely that drugs that inhibit one type of channel will also inhibit
others. Evidently, this may lead to side effects.
The structure of ion channels has been a common
cause of confusion. Ion channels must span the lipid membrane,
they must therefore have parts that stick out of the cell (which
can be targets for pharmaceutical intervention) and parts that
stick into the cell, permitting regulation by cell signalling
pathways.
The standardised structures of the alpha subunit
of a potssium and a sodium (or calcium) channel are given in
lecture 4 (and figure 1, below). Potassium, sodium and calcium
channels all exist as protein complexes. The alpha subunit contains
the pore of the channel and the voltage-sensor that allows the
channel to detect and gate in response to changes in the transmembrane
voltage. Recent work shows that the accessory subunits (variously
named as alpha2, beta, gamma etc.) might alter the behaviour
of the alpha subunit in a number of ways and may, in addition,
provide a way of anchoring the channel to the cytoskeleton, even
helping to target protein kinases to particular residues.
 |
Figure 1 - cartoon representation of the alpha (pore-forming) subunits of K channels and Na or Ca channels. |
It is strange but true that the amino acids that
line the pore of the voltage-gated channels are not those that
are found within the transmembrane domains. Research using many
different approaches has shown that the pore is formed by the
extracellular loop that links transmembrane domains 5 and 6 (see
figure 2 below).
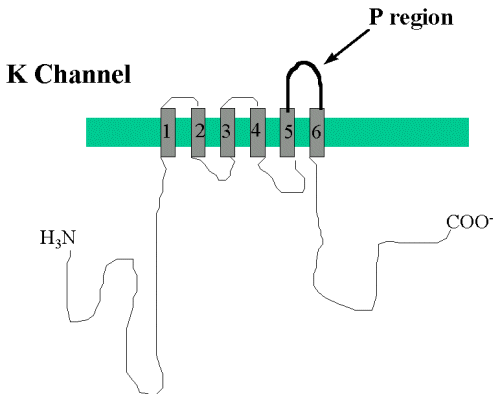 |
Figure 2 - Diagram to show in more detail the transmembrane domains numbered 1 thro 6 and the pore forming region. |
This portion between transmembrane domains 5 and
6 is sometimes termed the P region (P for pore). This
region is very highly conserved in all voltage gated channels.
Small changes in the amino acid residues within this region can
cause a Ca channel to loose its selectivity for Ca and become
a non-selective or even sodium selective channel. Indeed it is
possible using molecular biology to graft a P region from a Na
channel into a gene for a Ca channel (surplanting the normal P
region sequence) and produce a sodium selective channel with many
of the characteristics of a Ca channel.
The way that the P region dips into the membrane
to form the ion channel pore is shown diagramatically in figure
3.
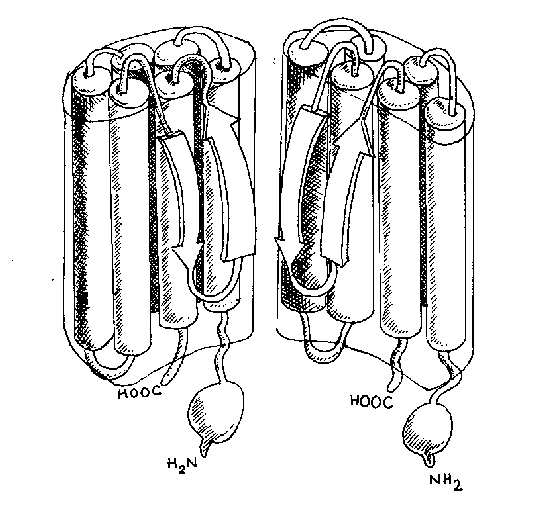 |
Figure 3 - Cartoon to show how the transmembrane domains of the alpha subunit form the ion channel pore (half of subunits are missing from this diagram) |

