Combatting antimicrobial resistance
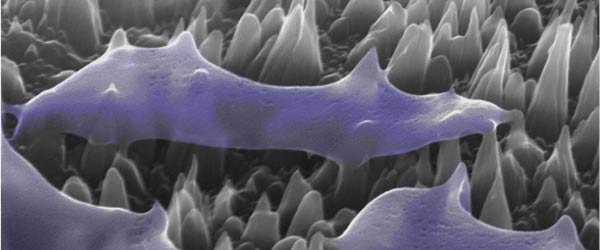
Microbiologists at the University of Bristol are investigating exactly why current drugs are not working in an attempt to reverse this trend before it’s too late.
Antibiotic resistance has been steadily increasing since the discovery of antimicrobials in the 1940s, as successive strains of bacteria evolve and adapt, rendering current therapeutic treatments redundant. Health institutes globally have warned of the catastrophic consequences if this cycle continues, with England’s chief medical officer Dame Sally Davies warning that without new drugs, “we will find ourselves in a health system not dissimilar to the early 19th century at some point”.
However, creating new antimicrobial drugs and discouraging the overuse of existing ones can only go so far. Understanding the biological reasons for antibiotic resistance in bacteria could also yield promising long-term results.
Dr Matthew Avison, a microbiologist for 15 years and a biochemist prior to that, works specifically with Gram negative bacteria, organisms that have been overshadowed by the focus on more commonly known Gram positive superbugs such as MRSA and C.diff. While these Gram positive pathogens have since been reduced to low numbers, the prevalence of Gram negative bacteria has been steadily increasing, posing a new risk to hospital patients in particular. For example, there are more than 25,000 cases of Gram negative sepsis, where the bacterium gets into the bloodstream. Currently, death rates are around 30 per cent and antibiotic resistance is a key factor in determining whether a patient dies or not.
One of the most important Gram negative bacteria is Klebsiella, ordinarily found in the human intestines and a common cause of infections such as pneumonia and sepsis in hospitals. This is something that Dr Avison’s lab in Bristol’s School of Cellular and Molecular Medicine has been working on for more than ten years – an indication of the necessarily lengthy process it takes for research to yield results.
“Our aim is to understand the mechanisms used by bacteria like Klebsiella to control the production of proteins involved in antibiotic resistance,” explains Dr Avison. “If we find out how bacteria switch on production of these proteins we might be able to stop it happening.”
Dr Avison’s lab works on bacteria taken from patients in hospitals around the world, using sophisticated comparative genomics, transcriptomics, proteomics, genetics and biochemistry methods. In the last five years, they have identified several proteins that are critical to controlling Klebsiella’s resistance to drugs, helping to piece together the biological map of the bacterium’s life-cycle. Another component of Dr Avison’s research involves developing an understanding of how to manipulate the bacterium’s outer envelope so as to increase its permeability, which would make it more responsive to antibiotics.
Understanding the various genetic components of bacteria that contribute to antibiotic resistance may also have the added benefit of allowing clinicians to personalise antibiotic treatment for each patient depending on which bacterial strain they are infected with, which could extend the life span of different antibiotics and reduce the chance of treatment failure.
Dr Avison adds: “Bacteria are very rarely resistant to every drug, so you need to be able to work out what resistance is present and then personalise the approach by prescribing a drug that the patient will best respond to and therefore benefit from. Within a few years I’m sure we’ll see rapid bedside diagnostics for identifying the bacteria causing an infection. If DNA technologies are used to do that, we can add the resistance elements we have discovered to the test, allowing a prediction of what drugs will and won’t work for a specific infection in a specific patient.”
The need for greater investment in and recognition of fundamental research cannot be understated, says Dr Avison: “Without this research, we will inevitably go back to a situation like it was before we developed antibiotics. A time when people routinely died of infections that can now be treated. The public and the authorities had, until recently, become blasé about the threat of infections; the assumption has been that people die of cancer or some other disease but not infections.
“The reality is that people do die of infections, in their thousands, normally because they have a weakened immune system, which is a side effect from some treatments, diseases, or just old age. But there will come a time when antibiotic resistance makes this problem even worse, and causes otherwise healthy people to start dying from infections. That’s where we’re going if we don't invest in fundamental microbiological research now. We cannot wait until then to start, because research takes a long time to make a difference.”
Related researchers
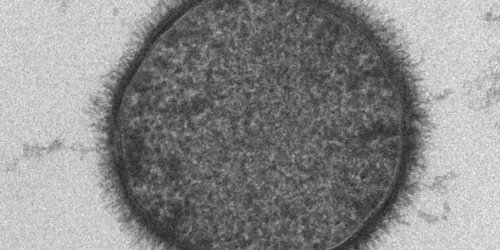 Study Cellular and Molecular Medicine
Study Cellular and Molecular Medicine
Contribute to the clinical application of scientific knowledge.