Study results
Title: Evaluating the effect of immunisation with group B meningococcal vaccines on meningococcal carriage.
Meningitis is a rare but potentially life-threatening infection around the surface of the brain. It mostly affects babies, young children, and adolescents.
A bacterium called meningococcus is an important cause of bacterial meningitis.
This study looked at whether immunising teenagers with a Meningitis B (MenB) vaccine could reduce the risk of others getting meningitis across the whole community.
‘Be on the TEAM’ meningitis vaccine study. We are excited to share the first results from the ‘TEAM’ study with you. MenACWY vaccines block throat carriage and will provide herd protection across the community. You can read the results at https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(22)00368-8/fulltext

COVID-19 Mapping and Mitigation in Schools (CoMMinS)
Title: COVID-19 Mapping and Mitigation in Schools.
The University of Bristol collaborated with Public Health England, the Bristol City Council, and local schools, to understand the practical challenges of preventing an outbreak of COVID-19 in schools.
Visit the CoMMinS study website for full details and results.
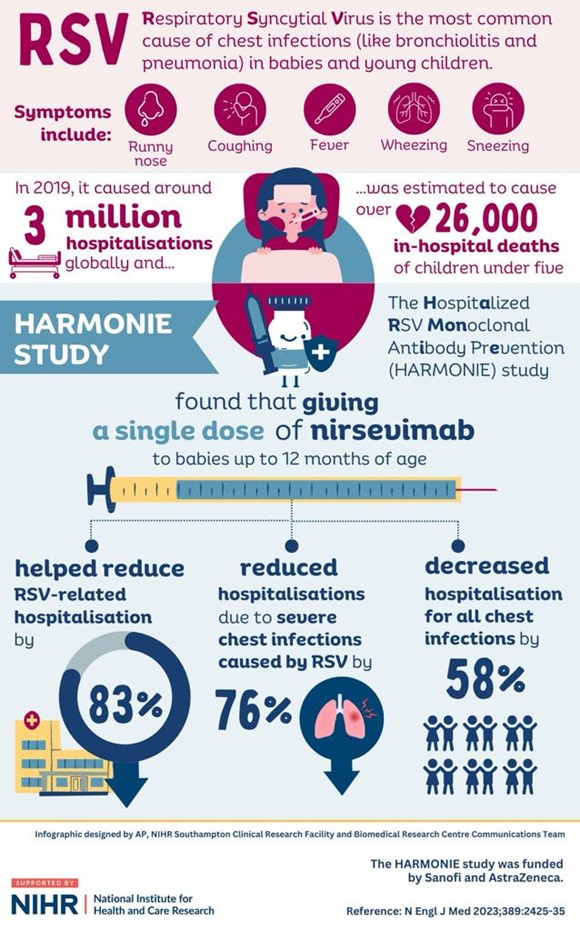
Harmonie Study
Title: A Phase IIIb randomized open-label study of nirsevimab (versus no intervention) in preventing hospitalizations due to respiratory syncytial virus in infants
The Harmonie Study found that Nirsevimab, the monoclonal antibody vaccine, delivers 83% reduction in RSV infant hospitalizations in a real-world clinical trial setting and reinforce its consistent and high efficacy against infant hospitalizations due to RSV.
Data presented at ESPID add to the body of evidence demonstrating nirsevimab’s protection against RSV-related lower respiratory tract disease (LRTD) and confirm its favourable safety profile in multi-country, real-world conditions.
Further information about the study results will be posted here.
MET52 - Study to evaluate conjugate MenACYW vaccine in the UK infant schedule
Title: Study to evaluate conjugate MenACYW vaccine in the UK infant schedule
All babies normally receive a vaccine against meningitis type B (MenB) as part of the UK vaccination schedule. The new vaccine (MenACYW) aims to protect against additional meningitis types A, C, Y and W. It has been tested in more than 5,700 people - babies, children, adolescents, adults, and the elderly - and found to be safe and protective against meningococcal disease.
However, the MenACYW vaccine is ‘investigational’ meaning that it is not yet licensed for use in the UK or elsewhere.
The aim of the study was to show whether the immune response to the MenACYW vaccine is as strong as is needed, to protect against meningococcal disease, when it is given alongside the childhood vaccines given routinely in the United Kingdom (UK). The study also looked at the safety of the MenACYW vaccine and the safety of the childhood vaccines when these are given together.
All participating babies received their routine vaccines as part of the study.
Please see our results here.
WHAT’S THE STORY
Title: What's the story? A Sero-epidemiological survey of England in 2019/2020/2021
The Bristol Children’s Vaccine Centre researched a new way of surveying how well protected we are from infectious diseases by collecting blood samples from people who represent different groups across society.
The study was run by Bristol Children’s Vaccine Centre, and the Oxford Vaccine Group (part of the University of Oxford).
Please see our results here.


