Aims of the study
There are three stages to the study covering our three main aims:
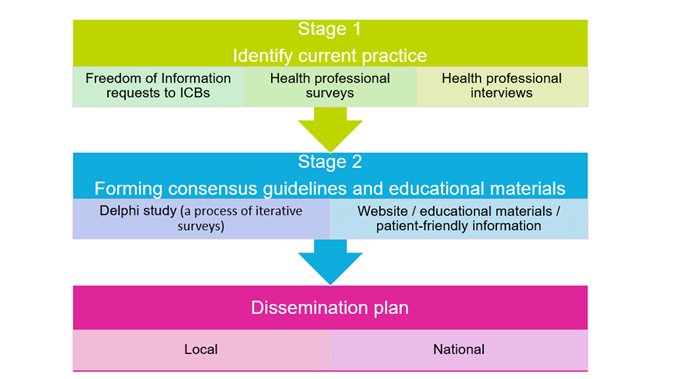
Stage 1: Identify current practice
- Find out how optometrists and hospitals in different areas of England manage people who may have nerve swelling.
- Identify where more training, equipment or resources might help.
Stage 2: Develop DIPP guidelines and educational materials
- Co-produce patient-friendly information leaflets with our patient and public involvement (PPI) group, who include a diverse range of members of the general public and patients with brain tumours.
- Develop consensus guidelines, educational resources, and a website for eye specialists and patients with The Royal College of Ophthalmologists and College of Optometrists.
- Trial the consensus guidelines with Bristol optmetrists and GPs and learn what they think about them.
Stage 3: Evaluate the impact of DIPP guidelines and educational materials
- Find out if Bristol optometrists and GPs adopted the guidelines and the impact on local NHS resources. We’ll discover whether patient experiences have improved as a result.
- Publicise the final consensus guidelines nationally to stakeholders, policymakers, charities and the public through organisations such as the Department of Health, NHS England, National Institute for Health and Care Excellence, Royal Colleges of Ophthalmologists, GPs & Physicians, and College of Optometrists. We’ll give talks at national meetings, publish articles and policy briefings in health and professional journals and the media.
Figure 1: Research methods by stage