Professor Zafar Bashir
Neurones of the brain communicate with one another at specialised junctions, called synapses, through the process of synaptic transmission. Having hundreds or thousands of synapses on individual neurones provides for immense complexity in neuronal signalling. However, a further degree of complexity comes about because the communication between neurones is extremely plastic. This means that the strength of synaptic transmission is not fixed but can be increased or decreased (a process called synaptic plasticity) and this property of synaptic plasticity is one of the crucial processes that enables the brain to learn and to retain memories. Therefore, understanding how synaptic transmission occurs and how it can be regulated and modified is essential if we are to understand the workings of the central nervous system and to understand the processes of learning and memory.
Glutamate is the main transmitter at synapses in the brain and glutamate performs its functions by acting on a variety of different receptors; activation of each of these produces different effects at the synapse. The function of glutamate is also modified by other transmitters, such as acetylcholine. Teasing apart the different roles that these different receptors play and how these different receptors and transmitters interact with one another to produce synaptic plasticity is one of my main interests.
For example, one of the current projects in the lab is investigating how NMDA receptors differ in their subunit composition between different pathways (perforant path and Schaffer collaterals) that terminate onto CA1 pyramidal neurones in the hippocampus. We are interested in understanding whether differences in NMDA receptors confer different plastic properties on the synapses at the two input pathways to CA1 neurones (see Figure 1).
To investigate synaptic transmission and plasticity I utilise a variety of in vitro techniques (molecular, electrophysiology, pharmacology) in the hippocampus, perirhinal and prefrontal cortex, depending on the precise questions. An example of this type of work is shown in Figure 1, below. In collaboration with other groups we can then use this knowledge to investigate how mechanisms of plasticity are involved in learning and memory; an example of this type of work is shown in Figure 2, below.
See also: Mechanisms of memory identified
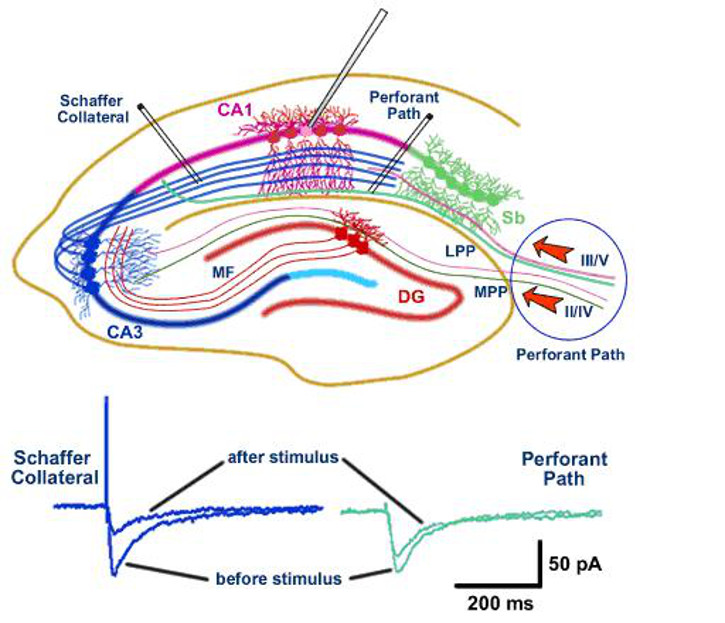
Figure 1. Plasticity of NMDA receptor EPSCs is different in different pathways (Schaffer collaterals v perforant path) that synapse onto CA1 pyramidal neurones in the hippocampus.
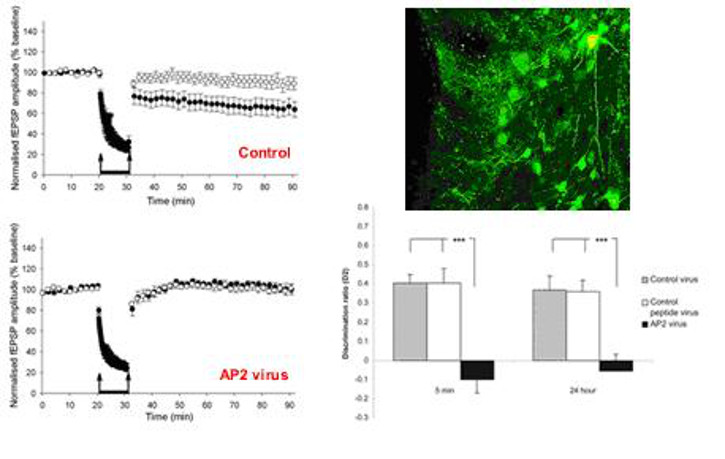
Figure 2. Expression of a peptide (visualised by EGFP; top right) that blocks the enzyme AP2, which is involved in receptor internalisation, abolishes LTD (bottom left) and disrupts visual recognition memory (bottom right).
Selected publications:
Derek Garden, Peter V Massey, Douglas A Caruana, Ben Johnson, E Clea Warburton, John P Aggleton and Zafar I Bashir (2009) Anterior thalamic lesions stop synaptic plasticity in retrosplenial cortex slices: expanding the pathology of diencephalic amnesia. Brain In Press
P. V. Massey, D. Phythian, K. Narduzzo, E. C. Warburton, M. W. Brown and Z. I. Bashir (2008) Learning specific changes in long-term depression in adult perirhinal cortex. J. Neurosci. 28, 7548-7554
Sarah Griffiths, Helen Scott, Colin Glover, Alison Bienemann, Mohamed T Ghorbel, James Uney, Malcolm W Brown, E Clea Warburton and Zafar I Bashir (2008) Expression of long-term depression underlies visual recognition memory. Neuron 58, 186-94.
Peter R Moult, Sonia AL Correa, Graham L Collingridge, Stephen M Fitzjohn, Zafar I Bashir (2008) Co-activation of p38 mitogen-activated protein kinase and protein tyrosine phosphatase underlies metabotropic glutamate receptor-dependent long-term depression. J.Physiol 586, 2499-510.
Jihoon Jo, Simon M Ball, Heon Seok, Seog Bae Oh, Peter V Massey, Elek Molnar, Zafar I Bashir* & Kwangwook Cho* (2006) Experience-dependent modification of mechanisms of long-term depression. Nature Neuroscience 9, 170-172. * Joint last authors.
Massey, PV., Johnson, BE., Moult, PR., Auberson, YP., Brown, MW., Molnar, E., Collingridge, GL., Bashir, ZI. (2004) Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J.Neurosci. 24, 7821-7828.
