![]() Sharpless
Asymmetric Dihydroxylation.
Sharpless
Asymmetric Dihydroxylation.
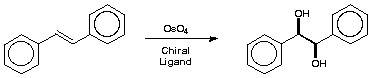 .
.
Introduction
This practical is partly derived from a paper¹ and those interested might like to look up a leading article² or even a review on the subject.³
A Note on Timing
After this reaction has been set up it needs to be left stirring overnight. It is ideal if you want to do another reaction while this one is going.
Osmium
Osmium tetroxide is extremely toxic. It is volatile which is increases its hazard and very expensive. In order to minimise the hazard to you, you will not handle osmium in the form of its tetroxide in this experiment. The osmium tetroxide is generated in situ from solid OsCl3 and then reduced at the end of the reaction. Solid OsCl3 is also extremely toxic and it has been weighed out for you into small vials which contain K2CO3 and between 9 and 10 mg of precious OsCl3.
The Reaction
This reaction has become one of the favourite ways of making enantiomerically pure materials due to its simplicity and generality. Unlike an ordinary dihydroxylation, which make racemic products, an asymmetric dihydroxylation selectively produces one enantiomer.
Notice the number of mmol of stilbene compared with the number of mmol of osmium that are used in this experiment.
Measuring the Enantiomeric Excess
You will do this using a polarimeter. It is an old method but still very effective (and most striking) with materials with high optical rotations.
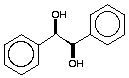
(1R, 2R)-1,2-Diphenylethane-1,2-diol. - Potassium ferricyanide (5.40 g, 16.4 mmol), potassium carbonate (2.26 g, 16.4 mmol), osmium (III) chloride hydrate (10 mg, 0.03 mmol) dihydroquinidine p-chlorobenzoate (100 mg, 0.27 mmol) and methanesulfonamide (0.53 g, 5.57 mmol) were added to tert-butyl alcohol (28 ml). Water (28 ml) was then added to the mixture which was stirred with a magnetic stirrer until all solids had dissolved. Stilbene (1.00 g, 5.55 mmol) was added and the suspension stirred overnight at room temperature. Anhydrous sodium metabisulphite (0.77 g, 4.05 mmol) was then added and stirring continued for 1 h before the addition of water (50 ml). The mixture was extracted with dichloromethane (3 × 50 ml) and the combined organic extracts were washed with 2 M KOH (5 ml), dried (MgSO4) and evaporated under reduced pressure. The residue was purified by flash chromatography, eluting with Et2O-hexane, , to give the diol (1.04 g, 87%).
Increasing the Enantiomeric Excess (ee).Measure the rotation and calculate the [a]D of your diol. , Recrystallise the diol. Measure the rotation and calculate the [a]D of your diol again. Calculate the ee of the diol from the reaction and the ee of the diol from after the recrystallisation. Repeat the recrystallisation
Write up. Record the IR and the m.p. of your recrystallised product.
In your write up you should identify the role that each reagent plays in
this reaction. Explain what recrystallising your product does. Find out
the cost of stilbene & the cost of enantiomerically pure hydrobenzoin
How much money did you make/lose in this experiment? What is the
melting point of the racemic compound? Interpret the IR & ¹H NMR
![]()