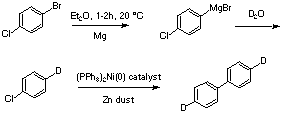
Background
The use of isotopically labelled compounds in organic chemistry has a long and distinguished history. The elucidation of many biosynthetic pathways¹ and organic reaction mechanisms has relied on the use of isotopes of predominantly hydrogen (²H - Deuterium, ³H - Tritium), carbon (12C, 13C, 14C) and oxygen (17O, 18O). Traditional methods have used 14C and 3H as radioactive labels. More recent methods have used the NMR properties of 2H and 13C as labels. In this experiment D2O is used as a source of 2H for the synthesis of a labelled biphenyl. The biphenyl has been used as an in situ measure of 2H concentration of 2H-labelled species during a mechanistic investigation of Pd-catalysed reactions.²
4-[²H1]-chlorobenzene. Under nitrogen, 4-bromochlorobenzene (19.1g, 0.1 mol) in anhydrous ether (35 ml) was added dropwise to a stirred suspension of Mg turnings (3g, 0.12 mol) in anhydrous ether (45 ml) at a sufficient rate to maintain reflux. After addition, reflux was continued to complete Grignard formation (if required). The mixture was cooled in an ice-bath. D2O (5.35 g, 0.27 mol) was added dropwise with vigorous stirring. The mixture was stirred for 30min, then filtered through a pad of dry silica-gel using a water-pump, washing through with a few small portions of ether. The filtrtate and washings were combined and concentrated in vacuo (care b.p chlorobenzene is 132 °C!). The residue was distilled at atmospheric pressure (20 cm Vigreux column or similar) collecting 3 fractions and giving 4-[²H1]-chlorobenzene as an oil (b.p. 132 °C, 5.2 g, 46 %).
4,4'-[²H2]-biphenyl.³ Zn dust (3.46 g, 53 mmol), NaBr (396 mg, 3.9 mmol), triphenyl phosphine (3.46 g, 13.2 mmol) and Nickel (II) chloride (172 mg, 1.32 mmol) were placed in a 50 ml three-necked RBF under nitrogen. DMF (16 ml) was added and the mixture heated to 60 °C (mantle) to give a black solution. The heating mantle was removed and 4-[²H1]-chlorobenzene (3.0 g, 26.4 mmol) in DMF (3 ml) added dropwise at such a rate as to keep temp at 60-70 °C (a heating mantle was used if the temperature dropped below 60 °C). After complete addition the reaction was stirred at 60 °C for 1h. The mixture was cooled to RT and poured into 100 ml water which was filtered through a pad of Celite. The washings were extracted with hexane (1 × 100ml). The hexane extract was washed with water (2 × 50 ml) then brine (1 × 50ml), then dried (MgSO4) and evaporated in vacuo. The solid product was sublimed (ca. 65 °C, 0.1 mmHg) onto a cold-finger to afford 4,4'-[²H2]-biphenyl 1.6 g (76%).
Obtain 1H nmr for the two products, and ask a demonstrator for ²H spectra. Also obtain IR and MS data. As part of your report assess the amount of ²H in each of the two compounds you made. You may also like to consider how this type of methodology could be used to make the following compounds.

![]()