![]() Alkylation
of chiral enolates - The Evans Method.
Alkylation
of chiral enolates - The Evans Method.
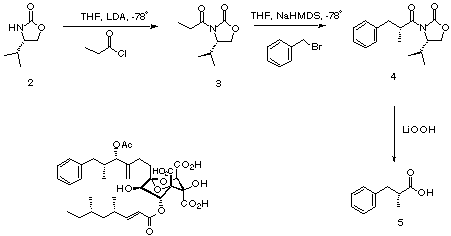
Squalistatin 1 [a]D = -27 c 1.65 in CHCl3¹
Background
Squalistatins are potent cholesterol biosynthesis inhibitors which have been isolated from fungal species. Drug companies are actively investigating this class of compound in the search for effective cholesterol lowering agents - a market with potential billion pound turn-over in the 'health'-conscious West. The biosynthesis of these compounds is also intriguing. In order to test whether R-2-methylphenylpropionic acid was a precursor to squalistatin 1, a synthesis was required to provide a source of isotopically labelled stereochemically pure material. One route would be through coupling of an enolate derived from ¹³C labelled propionate with benzyl bromide. Control of the stereochemistry of addition is necessary to provide the required R- configured chiral centre. The use of Evans chiral enolate technology² neatly allows the required control, and isotopically labelled R-2-methylphenylpropionate was indeed made this way.³
4S-3-[1'-oxopropyl]-4-isopropyl-2-oxazolidinone 3. To a stirred solution of S-4-isopropyl-2-oxazolidinone† , (1.0g, 7.75mmol) in anhydrous THF (30ml) at -78 °C was added nBuLi (1.1eqv, 2M in pentane, 4.3ml) dropwise over 2min. The solution was stirred for 20min at -78 °C, then propionyl chloride (1.2eqv, 9.3mmol, 860mg, 808µl) was added dropwise. The reaction was stirred for 1h, then allowed to warm to RT over 15min. The reaction mixture was poured into water (100ml) and extracted with EtOAc (3 × 100ml). Combined organic fractions were dried (MgSO4) and evaporated to afford a crude yellow oil. The title compound was obtained by flash column chromatography eluting with 15% EtOAc in 60/80 petrol.‡
Obtain IR, ¹H nmr and mass spectra. Interpret the results.
(4S, 2'R)-3-[2'-benzyl-1'-oxopropyl]-4-isopropyl-2-oxazolidinone 4. To a stirred solution of 4S-3-[1'-oxopropyl]-4-isopropyl-2-oxazolidinone (900mg, 5.8mmol) in anhydrous THF (10ml) cooled to -78 °C was added NaHMDS (1.1eqv, 1M in THF, 6.4mmol, 6.4ml) dropwise over 2min. After 30min a solution of benzyl bromide (1.1 eqv, 6.4mmol, 1.09g, 760µl) in anhydrous THF (2ml) was added dropwise. The reaction was stirred for 2h at -78 °C before the addition of saturated aqueous NH4Cl (10ml). The reaction was allowed to warm to RT, then poured into water (25ml). The mixture was acidified to pH 2 (conc. aq. HCl) and extracted into EtOAc (3 × 100ml). Combined organic fractions were dried (MgSO4) and evaporated to afford a crude yellow oil. The title compound was obtained by flash column chromatography eluting with 100% 60/80 petrol (100ml) followed by 15% EtOAc in 60/80 petrol.
Obtain IR, ¹H nmr, and mass spectra. A COSY nmr spectrum will be provided. Interpret the results.
![]()