![]() Enzyme
Catalysed Resolution of an Amino Acid.
Enzyme
Catalysed Resolution of an Amino Acid.
Background
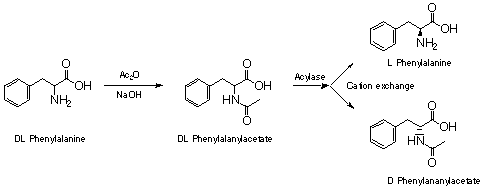
L-Amino acids are the building blocks of natural peptides and proteins. The synthesis of novel proteins and peptides as pharmaceuticals relies on the ready availability of natural and un-natural L-amino acids. Many synthetic methods are available for the synthesis of racemic amino acids (e.g. Strecker and Gabriel syntheses). In order to isolate pure L-amino acids the racemic materials must be resolved. One simple and cheap method relies on the use of Acylase to selectively hydrolyse L-amino acid acetates.¹ L-Amino acids and D-amides are then separated by cation exchange chromatography and recrystallised to purity. Isolated D-amides may be chemically converted to the corresponding amines by acid catalysed hydrolysis. Enzymes have found many other applications in modern organic synthesis.²
D-N-acetylphenylalanine and L-phenylalanine. A stirred suspension of DL-N-acetylphenylalanine (1.0g, 4.8mmol) in deionised water (25ml) was adjusted to pH 7.5-8 by the careful addition of saturated aqueous LiOH. Acylase (50mg) was added to the clear solution and the reaction was then incubated at 37 °C overnight. Decolourising charcoal (1g) was added with mixing and the black mixture filtered through celite. The celite pad was washed with deionised water (15ml). The combined aqueous washings were applied to the H+ form of a column of DOWEX AG50-WX8 cation exchange resin.
D-N-acetylphenylalanine - The column was eluted with deionised water until the pH returned to neutral. Evaporation of the aqueous washings afforded the title compound as a white solid. The thoroughly dry solid was recrystallised from EtOAc. Alternatively the product can be extracted from an acidified aq solution with EtOAc.
L-phenylalanine - After retrieval of the D-acetate, the column was eluted with 1N NH4OH in deionised water. Aqueous fractions were collected in test tubes and ninhydrin positive fractions were pooled and evaporated to afford the title compound as a white solid which was recrystallised from ethanol/water.
Column regeneration - Excess ammonia was washed away with deionised water. The column was regenerated by elution with 1N HCl in deionised water until pH<2. The column was then eluted with deionised water until the pH returned to neutral.
Obtain for each product : m.p., IR, ¹H nmr, aD. Calculate yields and
enantiomeric excesses. Interpret the spectra.
![]()