Background
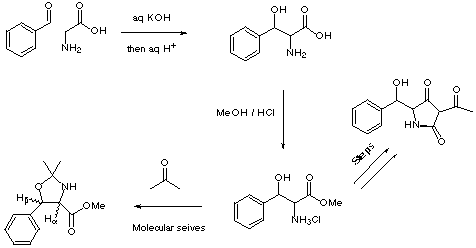
The reaction between aldehydes and glycine provides a convenient route to b-hydroxy-a-amino acids. The reaction is easy to perform, requiring the mixing of two equivalents of aldehyde with one equivalent of glycine in strong aqueous base, followed by acidic workup.¹ The amino-alcohols can be used in the construction of novel b-hydroxy tetramic acids, a class of natural products posessing biological activities. b-Hydroxy tetramic acids may also be biosynthetically related to 2-pyridones which are of potential pharmaceutical interest.² One way to determine the relative stereochemistry of the product is to lock the two heteronuclear substituents into a ring so that the geometry between the aromatic and ester groups is rigidly held. This can be achieved by forming the dimethyloxazoline with acetone. Unfortunately Ha-Hb coupling is not always diagnostic for geometry in five-membered rings,³ but an alternative NMR technique can be used. NOE enhancements between methyl protons and Ha and Hb will reveal the relative stereochemistry.†
Methyl 1-amino-2-hydroxy-2-phenylpropionate hydrochloride hemihydrate. b-phenylserine (1.0g, 5.53mmol) was dissolved in a solution of 10% HCl in anhydrous methanol. The solution was heated to reflux for 4h, cooled, and solvent evaporated in vacuo to afford the product as a colourless solid. The solid was recrystallised from ethanol/ethyl acetate to afford the product as a colourless crystalline solid (1.0g, 4.18mmol, 75.6%).
2,2-dimethyl-4-methoxycarbonyl-5-phenyl-1,3-oxazoline. Methyl 1-amino-2-hydroxy-2-phenylpropionate hydrochloride hemihydrate (100mg, 418µmol) was dissolved in anhydrous acetone (10ml) in a dry flask containing anhydrous 4A molecular seives. The mixture was heated to reflux for 30min, cooled, filtered to remove molecular seives and evaporation in vacuo afforded the required product in quantitative yield.
Obtain ¹H and IR spectra for each product. A NOE difference spectrum of the oxazoline will be provided. In your report interpret the spectra, determine the relative stereochemistry of the amino-alcohol and in the light of your conclusions comment on a likely mechanism for the aldol reaction.
![]()