![]() The
HornerWittig Reaction..
The
HornerWittig Reaction..
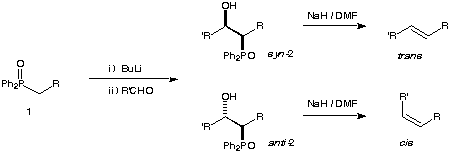
This experiment involves the preparation of a phosphine oxide which is deprotonated and reacted with an aldehyde to yield two diastereomers. This practical involves three reactions and one column.
Introduction
The Horner-Wittig reaction is a variation of the Wittig reaction and, unlike the Wittig reaction, allows the stereospecific preparation of double bonds.¹ Treatment of a phosphine oxide 1 with butyl lithium and then with an aldehyde yields a mixture of b-hydroxy phosphine oxides 2. These may be separated by chromatography and treated (separately) with sodium hydride. An anti b-hydroxy phosphine oxide yields trans alkenes and a syn b-hydroxy phosphine oxide yields cis alkenes.
The Reaction
In this experiment you will prepare a phosphine oxide - pentyldiphenylphosphine oxide. You will lithiate it and react this lithium derivative with anisaldehyde to prepare both syn and anti diastereomers of a ß-hydroxy phosphine oxide.
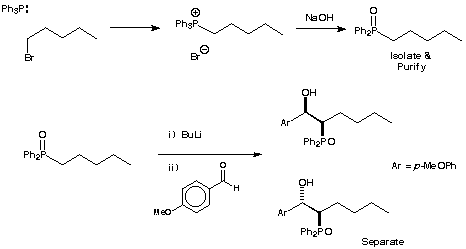
![]()
Pentyldiphenylphosphine oxide 3 :- Pentyl bromide (2.5 ml, 20.2 mmol) was added to triphenylphosphine (2.0 g, 7.63 mmol) and the mixture brought to reflux. After 5 minutes the reaction mixture had become extremely thick and it was left to cool. Toluene (5 ml) was added and the mixture brought to reflux for 30 min. The mixture was allowed to cool and the precipitated phosphonium salt filtered off and washed with toluene. The phosphonium salt was used in the next step without purification. A solution of NaOH (14 ml of 30 % w/w) was added to the phosphonium salt and the mixture was refluxed for 45 min. The mixture was cooled and extracted with CH2Cl2 (2 ×30 ml). The combined organics were washed with water (5 ml) and dried (MgSO4) and evaporated under reduced pressure to give the crude phosphine oxide (1.85 g, 89%). The phosphine oxide was recrystallised from EtOAc/40-60 pet. ether to yield the pure phosphine oxide (1.50 g, 72%).
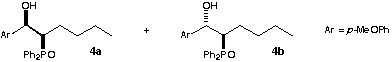
Both diastereomers of 2-Diphenylphosphinoyl-1-(4-methoxyphenyl)-hexan-1-ol 4a & 4b :- Pentyldiphenylphosphine oxide 3 (1.00 g, 3.68 mmol) was dissolved in dry THF (15 ml) and the solution cooled to -78 °C and n-butyl lithium (2.5 ml of a 1.6 M solution, 4.00 mmol) added dropwise. After 10 minutes, anisaldehyde (0.55 ml, 4.52 mmol) was added dropwise. The reaction mixture was stirred at -78 °C for a further 15 min before being allowed to warm slowly to room temperature and stirred for a further hour. Saturated ammonium chloride solution (30 ml) was added and the aqueous suspension extracted with CH2Cl2 (3 × 30 ml). The combined organics were dried (MgSO4) and evaporated under reduced pressure to give the crude product mixture (1.99 g). The mixture was purified by flash chromatography eluting with EtOAc-40/60 petroleum ether to yield (in addition to unreacted aldehyde) one diastereomer of the b-hydroxy phosphine oxide (1.03 g, 70.8 %) followed by the other diastereomer (0.27 g, 18.5 %).
Measure your yield of 4a and 4b before you recrystallise them.
Write up Submit recrystallised phosphine oxide 3 and phosphine oxide 4a & 4b for ¹H NMR. Record the IR and the m.p. of your recrystallised products. Interpret the IR. Submit your samples for mass spectral analysis.
Interpretation of the ¹H NMR. Use your ¹H NMR to work out which diastereomer was which from the reaction. You will need this information
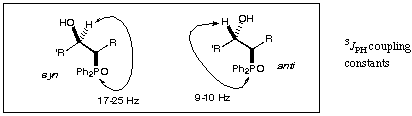
The coupling constant between the proton next to phosphorus and that next to the OH can often be very small.
Your write up should, of course, include a mechanism for the reaction.
![]()