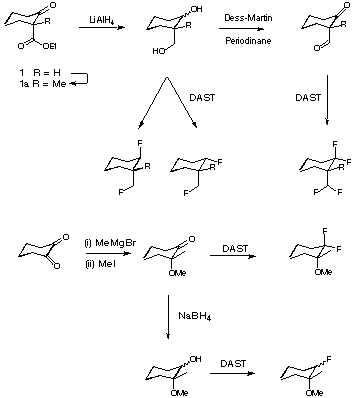
'Anomeric' Effects of Ring Difluoromethylene Units?
R. J. Cox
Fluoro and difluoromethylene units have been used as isosteres of oxygen in a range of compounds of potential pharmaceutical interest. The Van der Waals radius of F in the C-F bond is similar to H, and the short and highly polarised nature of the C-F bond may mimic oxygen lone-pairs. Additionally the introduction of a CHF or CF2 unit in place of O can confer stability towards aqueous hydrolysis of C-O bonds - especially in the pyran or furan positions of glycosides. In glycosides the anomeric effect is an important factor in determining the solution conformation of the ring moiety. Little is known about the potential for a C-F anomeric effect in cyclic compounds. Accepted wisdom regarding the anomeric effect in glycosides postulates orbital overlap between pyran oxygen lone-pairs and the polarised anomeric C-O bond. The lack of lone pairs in CF2 units could preclude this effect if there were poor overlap between the short polarised C-F bonds and the anomeric C-O or C-C bond s* orbital. In order to investigate the potential for C-F anomeric effects the following synthetic routes are under investigation. The resulting fluorinated cyclohexanes will be investigated by NMR methods to determine preferred conformations. The synthetic routes offer access to a range of compounds of interest.
