Harnessing microbial interactions in prevention and management of antimicrobial resistance
Investigating microbial biofilms and their potential for use in antimicrobial agents
What is the problem?
The National Institutes of Health (NIH) estimates that over 80% of all human infections are caused by structured microbial communities known as ‘microbial biofilms.’ Microbial biofilms are often formed by more than one type of microorganism, hence believed to be polymicrobial. The infections caused by polymicrobial biofilms are gaining significant attention due to their overwhelming impact on human health, quality of life and the healthcare economy. Particularly, polymicrobial infections that occur on indwelling devices within immunocompromised individuals and in healthcare settings are highly resistant to routine antimicrobial therapy, leading to alarming rates of treatment failure. The greater incidence of mortality and morbidity associated with these infections are due to increased dissemination behaviour of the pathogenic microbes, their enhanced antimicrobial resistance profiles, and the lack of sensitive diagnostics. Therefore, effective tools for early identification coupled with novel therapeutic approaches are quintessential to combat polymicrobial infections and the ever-rising threat of antimicrobial resistance worldwide.
A potential solution
The severity and outcome of polymicrobial infections can be predicted, not only by the microbial composition in the biofilms, but also by the specific interactions of the biofilm microbes. Pathogenic microbes deploy various molecules during microbial interactions. Dr Nihal Bandara (Bristol Dental School) and his team have been investigating the role of these molecules in developing biofilm antimicrobial resistance and the prospect of harnessing them as antimicrobial agents. Also, the team are exploring the potential of monitoring some of these molecules as ‘biomarkers’ for early detection of polymicrobial biofilm development and subsequent causation of infections.
An in depth understanding of these interactions in polymicrobial biofilms and infections, will likely facilitate optimisation of current antimicrobial regimes and, identification of antimicrobial molecules or unique cellular targets in designing novel therapeutic strategies.
Outcome and next steps
From the interdisciplinary research conducted, Dr Bandara and the team identified the impact of specific bacterial chemical signalling molecules in developing antifungal resistance in co-inhabiting yeast pathogens. They also demonstrated a promising use of some of these signalling molecules as adjuncts to current antibacterial and antifungal drugs to achieve better elimination of pathogenic biofilms. Further investigations are currently underway in discovering molecules from polymicrobial biofilms for early detection of infections occurring in indwelling devices.
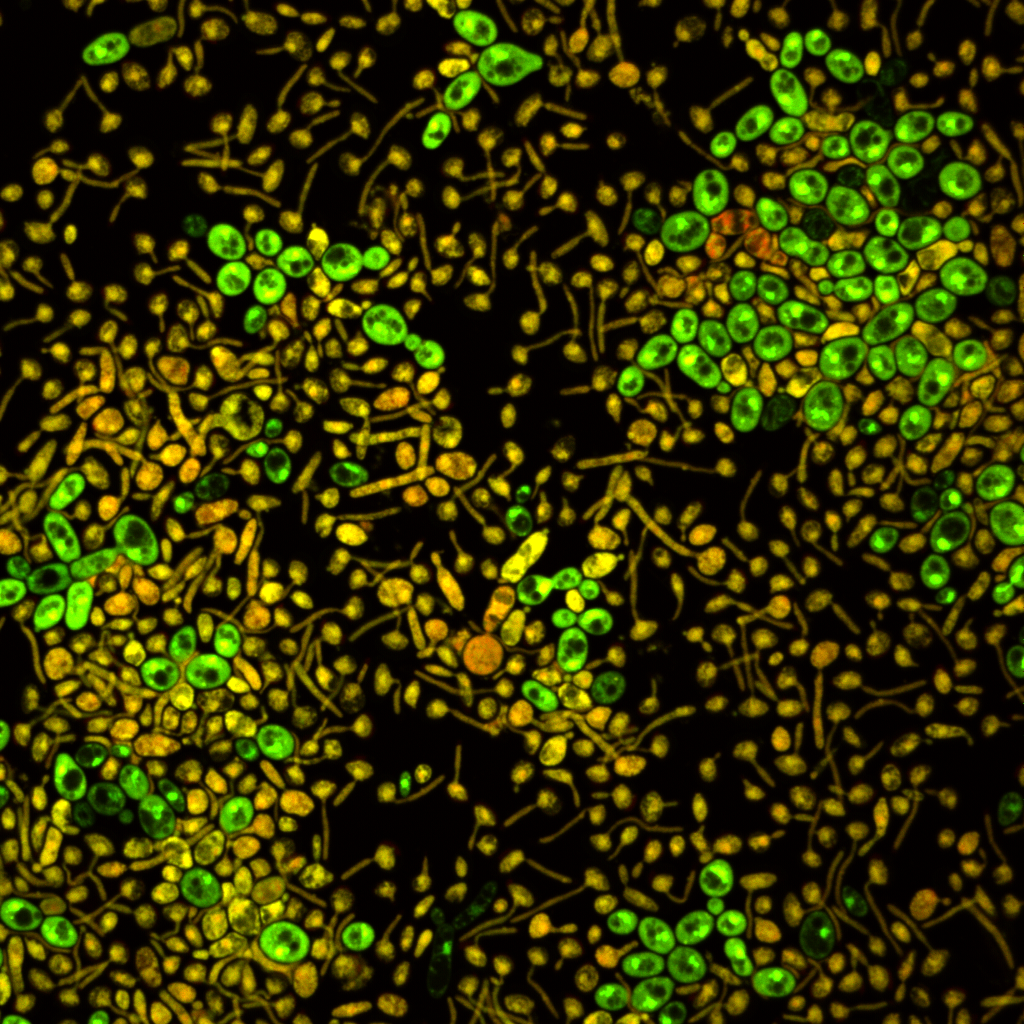
Researchers involved
- Dr Nihal Bandara (Bristol Dental School)
- Dr Angela Nobbs (Bristol Dental School)
- Dr Mark Jepson (Wolfson Bioimaging facility)
- Dr Jonathan Tyrrell-Price (University Hospitals Bristol)
- Dr Rajeka Lazarus (University Hospitals Bristol)
External collaborators
- Prof Elizabeth Johnson (UK National Mycology Reference Laboratory)
- Dr Andy Borman ((UK National Mycology Reference Laboratory)
- Prof Hugh Smyth (The University of Texas at Austin, USA)
- Prof Philip Hugenholtz (The University of Queensland, Australia)
- Prof Lakshman Samaranayake (The University of Hong Kong, Hong Kong)
- Dr Paul Tsang (Technological and Higher Education Institute, Hong Kong)
Funding
- Above and beyond – University Hospitals Bristol
- International Association for Dental Research
Press releases and useful links
- https://www.bristol.ac.uk/people/person/Nihal-Bandara-803915f0-3dc3-46b1-a801-12d71259ed4c
- https://www.bristol.ac.uk/news/2020/april/candida-infections.html
- https://www.bristol.ac.uk/dental/news/2018/nihalbandara.html
- https://www.nature.com/articles/s41598-020-64761-3
- https://www.sciencedirect.com/science/article/pii/S0378517320300806?via=ihub